Callionymidae: D III A3
Callionymus marleyi Regan 1919, and ?Synchiropus sp
Sand dragonet


Egg diameter in µm |
Number of oil globules |
Diameter of oil globule in µm |
Yolk texture |
Perivitelline space |
Position of oil globule at hatch |
Gut length at eye- pigment stage |
Myomeres |
680-740 |
0 |
n/a |
segmented |
narrow |
n/a |
50% of NL |
22 |
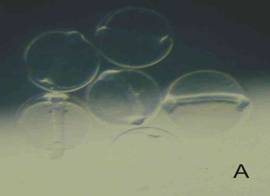
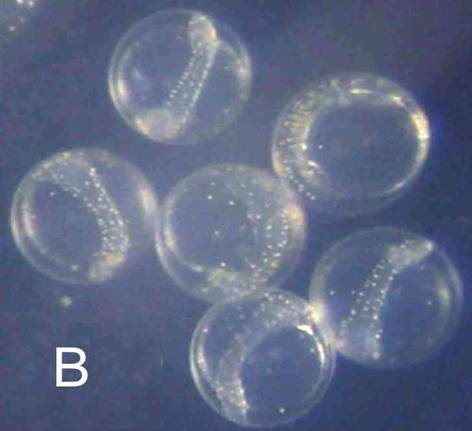
Egg: When fresh, the small size, coarse segmentation of the yolk, and lack of an oil globule (A ), set this egg apart from all others found in the area, except for DIIIA1, a phosichthyid`, CDIIIA1 (reticulated chorion) and DIIIA4 (flat spots on chorion). As the egg develops, both the embryo and yolk surface become covered with yellow-brown spots (B), whereas the clupeiform develops into an elongate embryo without yellow pigment. Eggs hatched in about 25 hours (23°C).
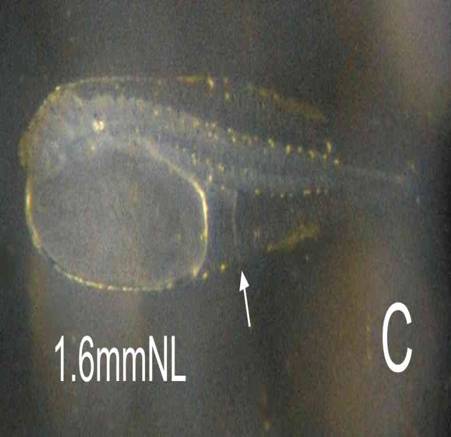
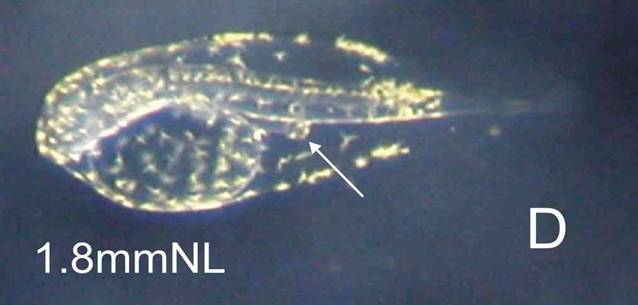
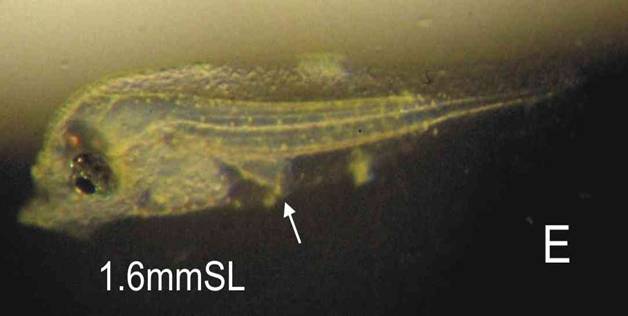
Larva: The newly hatched larva is compact, and is peppered with yellow-brown pigment dots, more concentrated along the edge of the finfolds (C). This finfold pigment remains with the larva through to 6 days, concentrating in 4 patches (F). Eye pigment and a functional mouth are present at 4 days (E). C: NH, D: 1 day, E: 3 days, F: 6 days, G: 42 days.
This was the smallest egg I managed to rear, mainly because the first-feeding larva (4-6 days) has a generous mouth. From a batch collected in November 1988, and successfully reared, the three surviving juveniles settled over 2 days, 28-29 days post-hatch. Six larvae have been barcoded, 4 from one sample, and two from separate samples, and submitted under the egg code CDIIIA1. Five cluster as one species in the barcode dendrogram, but do not match any adult sequences in BOLD, including a single specimen of Callionymus marleyi, while 1 matches Dracula celetus. I have barcode 8 of the 10 species listed by Fricke (1986), and have barcodes of an additional 4 and possibly 5 other callionymid species from eggs and larvae collected in plankton samples at Park Rynie, but this species remains unidentified by barcoding. I have retained C. marleyi on this page because I reared it from this egg, but the barcode identification remains uncertain as no larvae from eggs have yet provided a match with C. marleyi, the barcode of which is also tentative, being based on a single specimen.
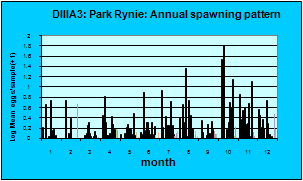
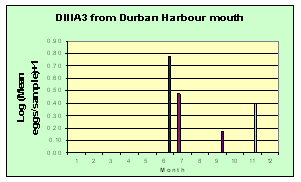
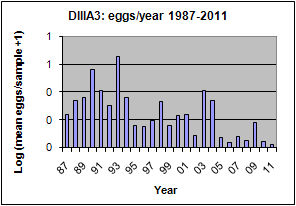
This was one of the more common eggs in Park Rynie samples (Introductory notes: Section 7, Table 3), with some 1500 taken during the study. The species spawned all year round, with a spring and early summer maximum (blue graph). The egg was seen in the DHM samples on only four occasions (green graph). Catches off Park Rynie show a progressive drop over the period of the study (white graph). The Park Rynie linked samples had 63% of the eggs offshore, suggesting spawning occurs inshore of the two indicator species, around the 30m depth contour. See Section 7.3 and Table 1 of the Introductory Notes, for more information on the linked samples.
Linked samples |
Offshore |
Inshore |
Eggs |
625 |
366 |
Hits |
77 |
137 |